Ammonia cracking is gaining significant attention as the key that enables the long-distance transport and storage of hydrogen. In this Q&A, Topsoe expert Maninder Jit Singh explores the fundamental aspects of ammonia cracking, including its significance, the technology behind it, its role in the hydrogen economy and how it can be a great business opportunity. So, let's get cracking.
What is ammonia cracking?
Ammonia cracking is a chemical process that converts ammonia (NH3) into its constituent elements, nitrogen (N2) and hydrogen (H2). This reaction is typically carried out at high temperatures and can be catalyzed by various catalysts. The ammonia cracking reaction can be represented by the following equation:
2NH3 ⇌ N2 + 3H2
The process is endothermic, meaning it requires the input of heat energy. The catalyst helps to lower the activation energy required for the reaction to occur, thus increasing the reaction rate.
The most basic layout for an ammonia cracking process includes at least the following steps: Vaporization and preheating of the raw ammonia feed, catalytic decomposition of ammonia, and the removal of unconverted ammonia and purification of the hydrogen product.
What is ammonia cracking used for in industry?
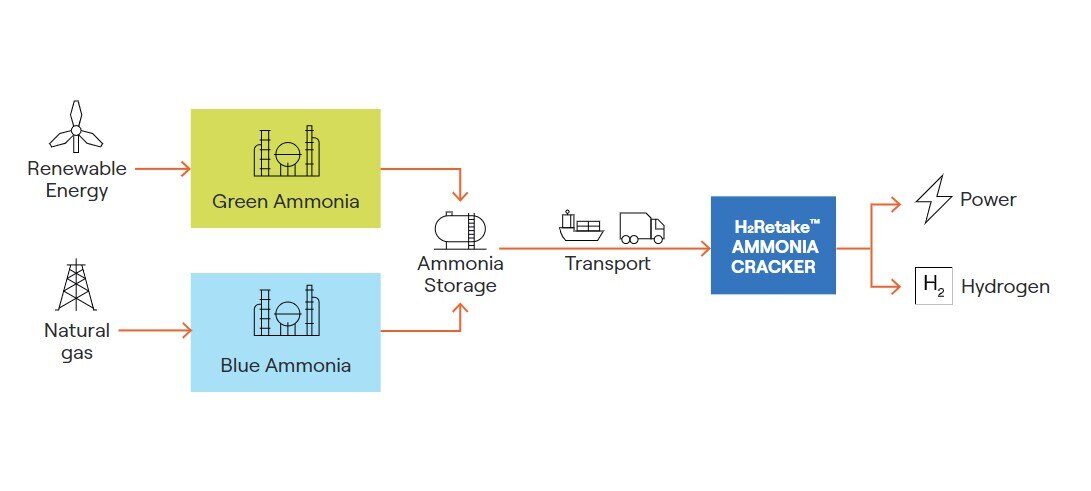
Ammonia cracking is an important industrial process with several applications. One of the main uses is in the transportation of hydrogen gas, which is a valuable fuel and feedstock for various industries. Hydrogen can be turned into ammonia, a derivative product via the Haber-Bosch synthesis, explained later. It is then transported as ammonia and then converted back into hydrogen through ammonia cracking. The hydrogen can then be used can be used in fuel cells, for natural gas replacement and in various chemical processes.
Is the technology proven and available at large scale?
The technology used in large-scale ammonia cracking is a proven technology with decades of industrial use. Topsoe’s first ammonia cracking technology was developed back in 1978 with a focus on heavy water production. The largest ammonia cracking facility was built in Argentina in 1993, with a capability of cracking 2 x 2,400 MT per day of ammonia in two parallel lines.
Ammonia cracking can be centralized, large- to mega-scale with hydrogen transported to the end-use in extended hydrogen grids, such as those planned in Europe. It can also be decentralized, large-scale and co-located with large hydrogen off-takers possibly via local hydrogen grids. Finally, it can also be small-scale and decentralized, for example at hydrogen filling stations.
Why is ammonia important as a transportation method for hydrogen?
Ammonia is considered an important transportation method for hydrogen due to several reasons.
First off, it is energy dense. Ammonia has a higher energy density compared to compressed hydrogen gas. This means that a larger amount of energy can be stored and transported in the form of ammonia compared to compressed hydrogen, making it more efficient for long-distance transportation. Ammonia is free of carbon and, as such, is the highest energy density, non-carbon medium.
Crucially, Ammonia can be stored and transported as a liquid at -33°C, which means it does not require high-pressure or cryogenic storage. Ammonia is also easier to handle and has well-established safety protocols and regulations.
The second reason is that ammonia infrastructure is mature, and safety best practices already exist. Ammonia has an extensive existing infrastructure for production, storage, and transportation. It is already produced in large quantities for various industrial applications, such as fertilizer production. Leveraging this existing infrastructure can help facilitate hydrogen transportation without needing significant new infrastructure development.
Finally, the process to convert ammonia back into hydrogen through ammonia cracking is straightforward and proven on a large scale. This means hydrogen can be extracted from ammonia at the point of use, enabling the utilization of ammonia as an ideal and flexible hydrogen carrier.
What are the stages involved in using ammonia to transport hydrogen?
Hydrogen needs to be transported to different regions for several reasons. For instance, regions with high energy demand may not have sufficient local hydrogen production capabilities, while other regions have cheaper or more reliable feedstock. Regions rich in renewable energy sources (like wind or solar), for example, can produce green hydrogen through electrolysis. Transporting this green hydrogen to other areas supports the integration of renewable energy into the broader energy system.
Using ammonia as a carrier allows the end point user to take advantage of cheaper or more readily available feedstocks and production processes elsewhere on the globe. Meanwhile, ammonia’s ability to be liquefied allows it to be transported at a lower cost that hydrogen. These benefits help offset the costs of conversion involved and make the process efficient economically.
There are three main stages in transporting hydrogen using ammonia as a carrier. They are the initial conversion of hydrogen to ammonia, the transportation of ammonia, and lastly the conversion of ammonia back to hydrogen at the destination.
Here are the steps involved:
Conversion of hydrogen to ammonia
Hydrogen gas (H2) is first converted to ammonia (NH3) through a process called Haber-Bosch synthesis. This process involves reacting hydrogen with nitrogen gas (N2) under high pressure and temperature in the presence of a catalyst. The resulting ammonia is then liquefied and stored for transportation
Transportation of ammonia
Ammonia is transported using existing infrastructure such as pipelines, ships, or trucks, as it is already produced and transported in large quantities on global scale. The ammonia can be stored and transported as a liquid at -33°C, making it easier and safer compared to transporting hydrogen, which requires high-pressure or cryogenic storage.
Conversion of ammonia back to hydrogen
At the destination, the stored ammonia is converted back to hydrogen through ammonia cracking. The process begins by heating and evaporating liquid ammonia, which is initially at -33°C. This step requires the application of heat, approximately 23 kilojoules per mole of ammonia for the evaporation. Once vaporized and heated, the ammonia is then directed to the cracking section for ammonia cracking to occur.
In ammonia cracking, the ammonia vapor is further heated to high temperatures in the presence of a catalyst. This causes the ammonia to decompose into nitrogen gas (N2) and hydrogen gas (H2) according to the reaction: 2NH3 ⇌ N2 + 3H2.
The hydrogen produced from the ammonia cracking can then be used for various applications, such as in fuel cells, for natural gas replacement and in various chemical processes. This hydrogen can be stored, distributed, and utilized in the same way as hydrogen produced from other sources.
What are the critical areas to be aware of when considering ammonia cracking as a new business opportunity?
1. Process efficiency: Technology choice
The cost of the ammonia feed is the dominating cost of hydrogen from ammonia cracking. It is, therefore, of primary importance that the technology is the most efficient and optimized available. Any losses or inefficiencies will directly affect the hydrogen yield and result in extra energy-related costs. It is important to optimize the ammonia cracking process to minimize losses and maximize the hydrogen yield.
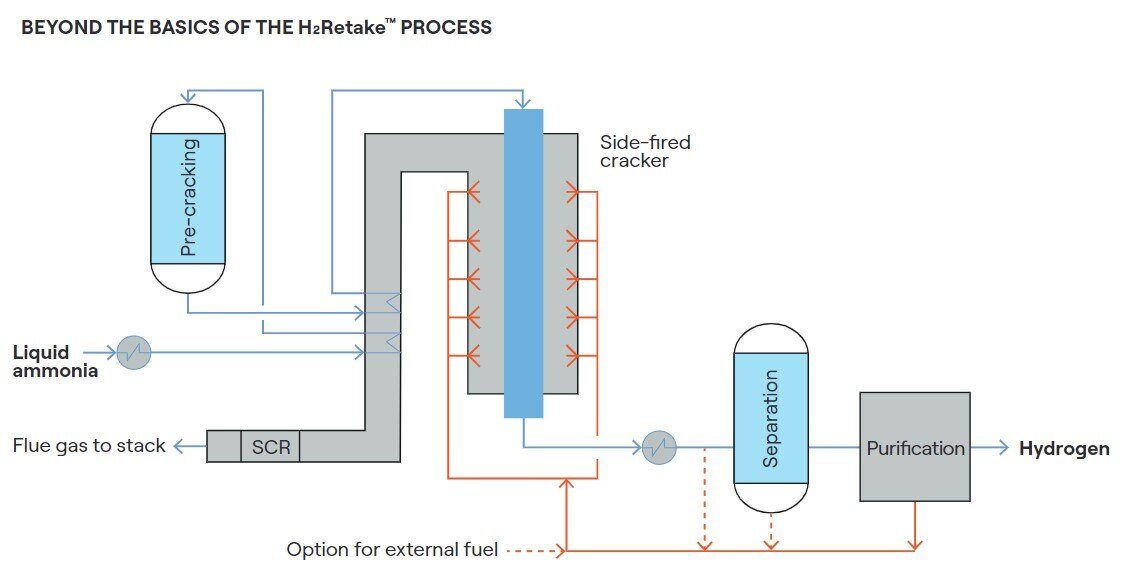
Topsoe’s technology, H2Retake (see diagram below), for instance, utilizes the energy input in the side-fired ammonia cracker to drive the decomposition reaction and effectively preheat and evaporate the raw feed with minimal wastage. The efficiency is achieved through a streamlined ammonia cracking design, optimized heat and off stream integration and careful catalyst selection. The result is an impressive energy efficiency of 96%.
2. Catalyst selection
The choice of catalyst for ammonia cracking is crucial. Selecting a catalyst that is highly active, stable, and with high strength and minimum pressure drop is essential for an effective and energy-efficient process. Understanding the performance and durability of the catalyst under different operating conditions is important to ensure optimal conversion rates and minimize catalyst degradation.
3. Safety: location and permitting
Ammonia is both toxic and corrosive, but it is also a long and widely used substance. As a result, safety procedures are well-established. These ensure proper safety measures are in place for the storage, handling, and transportation of ammonia. Assessing and implementing appropriate safety protocols, materiality of infrastructure and training is critical to mitigate any potential risks associated with ammonia handling.
The location of ammonia cracking facilities can be important in permitting approval. For instance, ammonia handling at ports is often mature and the surrounding safety ecosystem – from handling infrastructure, communications accessibility, security and emergency response protocols – is regularly in place. Locating facilities inland can mean additional complexities, especially if close to population centers.
4. Carbon intensity
Depending on the sustainability goals of the project, environmental impact and carbon intensity should be considered. The source of energy used for the process can impact the overall carbon footprint. Evaluating the environmental implications and exploring the use of renewable or low-carbon energy sources for ammonia production and cracking can help minimize the environmental impact. For instance, green methods that use renewable energy, result in the lowest environmental impact, while blue/low-carbon methods can result in low or very low carbon intensity, depending on the efficiency of carbon capture and potential upstream emissions.
5. Fuel choice for ammonia cracking
The choice of fuel for ammonia cracking is important for several reasons, each contributing to the overall efficiency, environmental impact, and economic viability of the process. The economic viability of ammonia cracking is influenced by the cost and availability of the fuel, so if the ammonia itself is being used, it is important that this usage is as efficient as possible to maximize the amount of hydrogen. Using ammonia as fuel decreases hydrogen yield but ensures that all process energy originates from ammonia, leading to a hydrogen product with zero carbon intensity (provided the ammonia has zero carbon intensity).
Utilizing external fuels boosts hydrogen yield but may cause CO2 emissions. However, using carbon-neutral fuels such as biogas or biomethane, such CO2 emissions will not impact the product hydrogen carbon footprint and can help aligning with environmental regulations and sustainability goals.
6. Knowhow and experience
Ammonia cracking is a growth area with many new players involved. Working with experienced technology providers means you have a partner with a deep understanding of ammonia cracking processes, catalysts, and reactor design, accumulated over years of research, development, and practical application.
This expertise and proven track record of successful implementations allows them to offer valuable insights, optimize the process, and troubleshoot any challenges that may arise during the project. Established technology providers often offer reliability and performance guarantees and have well-established project management processes and support systems in place covering the entire project lifecycle.
.png) Topsoe Academy™ Topsoe Academy™ is your chance to tap into over 80 years of accumulated knowledge Find out more
Topsoe Academy™ Topsoe Academy™ is your chance to tap into over 80 years of accumulated knowledge Find out more Discover how we meet the new energy reality to drive a sustainable future. Read
Discover how we meet the new energy reality to drive a sustainable future. Read There’s a skill in making the complex simple, and the simple exciting.The science of excitementRead
There’s a skill in making the complex simple, and the simple exciting.The science of excitementRead
.png)













